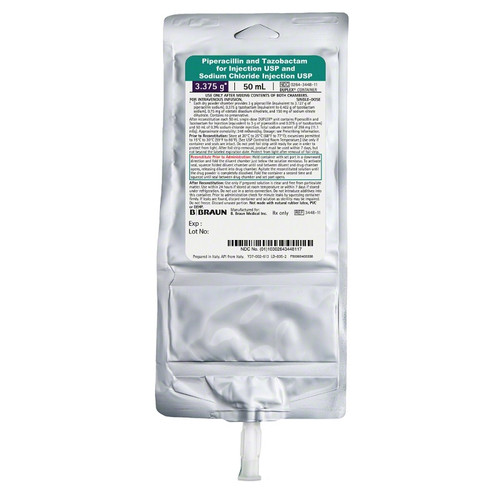
- Used as a combination penicillin-class antibacterial and beta-lactamase inhibitor for treating various bacterial infections
Uses
- Intra-abdominal Infections:
- Specifically appendicitis (complicated by rupture or abscess) and peritonitis
- Pneumonia:
- Indicated for nosocomial (hospital-acquired) and community-acquired pneumonia of moderate-to-severe severity
- Skin and Skin Structure Infections:
- Used for cellulitis, cutaneous abscesses, and ischemic/diabetic foot infections
- Female Pelvic Infections:
- Including postpartum endometritis and pelvic inflammatory disease
Features
- DUPLEX® Drug Delivery System:
- A dual-chamber, flexible IV bag that stores the drug (powder) and diluent (0.3% Sodium Chloride) separately until activation
- Ready-to-Activate:
- Requires no manual mixing, thawing, or special equipment; it is activated at the patient's bedside by folding and squeezing the bag
- Dosing Accuracy:
- Pre-filled with an exact dose of 3.375g (3g piperacillin and 0.375g tazobactam) and 50mL of diluent
- Integrated Safety:
- The container is not made with PVC, DEHP, or natural rubber latex and features a barcode that identifies the final mixed drug to ensure administration accuracy
- Convenient Storage:
- Can be stored at room temperature, saving pharmacy space compared to frozen or refrigerated options
Benefits
- Reduces Medication Errors:
- By eliminating manual compounding and complex reconstitution steps, it significantly lowers the risk of calculation or mixing errors
- Improved Efficiency:
- Reduces nursing labor time by up to 35% compared to other systems and has a faster reconstitution time than traditional compounding
- Enhanced Infection Control:
- The closed system prevents contamination during preparation and protects healthcare workers from exposure to airborne drug particles
- Regulatory Compliance:
- Designed to meet USP <797> guidelines for sterile compounding and The Joint Commission safety standards
Contents
- 3.375g Piperacillin and Tazobactam for Injection in 0.3% Sodium Chloride for Injection in 50mL DUPLEX Container
- 24 Containers per case